One day I shall learn to write short articles, but not today.
I make no apology for this one, despite being also off-topic: it’s too important to be condensed.
Has much of the world failed to benefit from an effective, early-stage treatment for Covid-19, because early trial results were misleading? There may be a number of drugs for which this might be questioned: here I look at hydroxychloroquine.
The Early Indications
Hydroxychloroquine is not an exotic new drug with which doctors and medical authorities have little experience. On the contrary it has been used widely for decades to treat malaria, lupus and rheumatoid arthritis. It came to public attention as a potential treatment for Covid-19 early in 2020, not least because of President Trump’s espousal of it.
In the period March – July 2020, attention focused on the WHO-led multinational Solidarity Trial and the UK’s own Recovery Trial which addressed the efficacy of hydroxychloroquine against Covid-19.
The Chief Investigators of the Recovery project released a press statement on 5 June 2020 which stated simply, “no clinical benefit from use of hydroxychloroquine in hospitalised patients with COVID-19”.
On 4 July 2020 the Solidarity project discontinued the hydroxychloroquine and lopinavir/ritonavir trials. The interim trial results showed that hydroxychloroquine and lopinavir/ritonavir produced little or no reduction in the mortality of hospitalized COVID-19 patients when compared to standard of care. The Solidarity Trial found that all four treatments evaluated (remdesivir, hydroxychloroquine, lopinavir/ritonavir and interferon) had little or no effect on overall mortality, initiation of ventilation and duration of hospital stay in hospitalized patients.
The Recovery and Solidarity trials were exclusively carried out on seriously ill patients in hospital, rather than the early-stage patients for which there was existing evidence that hydroxychloroquine might be effective. A drug which acts against the pathogen is most relevant when the pathogen is multiplying. In the later stages of Covid-19, the illness becomes an immune-system-driven inflammatory condition, and by that time the original pathogen has already done its damage. Could it be that the negative results of the Recovery and Solidarity trials were due to their deployment to patients in an inappropriate phase of the disease? Certainly, Professor Didier Raoult from IHU-Marseille, and an early leading proponent of hydroxychloroquine, was not impressed with the Recovery trial, accusing it as being “the Marx Brothers doing science”.
In passing I note that a further multinational trial, REMAP-CAP, was also deployed only to seriously ill patients with severe pneumonia admitted to an intensive care unit (ICU). I have found no results from this study. On 3 June 2020 it was suspended following the scare from a now infamous Lancet paper by Mehra et al which claimed the use of hydroxychloroquine increased death rates (the paper was retracted a few days later). I presume that trial was never restarted.
Another criticism of the Recovery and Solidarity trials which has been made is the dosage regime, the doses appearing to be substantially greater than standard practice when the drug is used against malaria, lupus or rheumatoid arthritis (see, for example, Killing the cure: The strange war against hydroxychloroquine).
Recent Evidence that Hydroxychloroquine is Effective Against Covid-19
Care is need here. To recommend a particular drug to medical practitioners requires a randomized controlled trial (RCT). However, before one goes to the expense and trouble of carrying out a controlled trial there must be some prima facie evidence to suggest a positive outcome is likely. Is there such prima facie evidence that hydroxychloroquine is effective when used in the right dosage on patients in the right stage of the disease?
Yes, there is. Lots of it. In fact, far more than is necessary to establish a prima facie case. It is surprise of this fact, against indications from the earlier trials, that motivated the writing of this short article. The number of studies emerging which examine the efficacy of hydroxychloroquine, sometimes in combination with other drugs, is substantial – far too many papers for me to review here. Fortunately review articles are now appearing which bring the big picture together, although what is still needed is a definitive, rigorous meta-analysis published in a top journal.
What I shall do first is give the reader a brief impression of what data is around by looking at a small sample of studies. These alone are sufficient to provide a solid prima facie case. You will see that the evidence comes from all over the world. The weight of evidence that hydroxychloroquine, possibly combined with other drugs, is efficacious in the early stages of Covid-19 is very strong. I will give a status summary of all the studies available as of February 2021 after looking at a few individual studies.
Didier Raoult and the Marseille Team
Although invariably associated in the press with the name of the flamboyant Didier Raoult alone, the paper which was submitted to the journal Travel Medicine and Infectious Disease on 27 May and published online on 25 June 2020 included the names of 43 authors: “Outcomes of 3,737 COVID-19 patients treated with hydroxychloroquine/azithromycin and other regimens in Marseille, France: A retrospective analysis”. Extracts from the Abstract are,
“In our institute in Marseille, France, we initiated early and massive screening for coronavirus disease 2019 (COVID-19). Hospitalization and early treatment with hydroxychloroquine and azithromycin (HCQ-AZ) was proposed for the positive cases. We retrospectively report the clinical management of 3,737 screened patients, including 3,119 (83.5%) treated with HCQ-AZ (200 mg of oral HCQ, three times daily for ten days and 500 mg of oral AZ on day 1 followed by 250 mg daily for the next four days, respectively) for at least three days and 618 (16.5%) patients treated with other regimen (“others”).
The patients’ mean age was 45 (sd 17) years, 45% were male, and the case fatality rate was 0.9%…..Treatment with HCQ-AZ was associated with a decreased risk of transfer to ICU or death (Hazard ratio (HR) 0.18 0.11–0.27), decreased risk of hospitalization ≥10 days (odds ratios 95% CI 0.38 0.27–0.54) and shorter duration of viral shedding (time to negative PCR: HR 1.29 1.17–1.42)…..Although this is a retrospective analysis, results suggest that early diagnosis, early isolation and early treatment of COVID-19 patients, with at least 3 days of HCQ-AZ lead to a significantly better clinical outcome and a faster viral load reduction than other treatments.”
Although many patients were relatively young in Covid terms, 12.3% were over 64 (5.7% older than 74). Of patients given hydroxychloroquine, 359 were over 64; of patients given other treatments, 157 were over 64. There was a high level of comorbidity: cancers 4%, diabetes 8%, chronic heart disease 6%, hypertension 15%, chronic respiratory disease 9%, obesity 11%.
The vultures have been circling Raoult for some time. The New York Times did a hit piece on Raoult in which “Trump” occurred 11 times. The Guardian was delighted to give Raoult some publicity…when the story was that he was to be subject of a disciplinary hearing by his professional medical body. In the Guardian story, “Trump” appeared in the third sentence. There were 42 other authors responsible for the paper as well as Raoult, but Trump was not one of them.
Bernaola (Madrid)
The paper Observational Study of the Efficiency of Treatments in Patients Hospitalized with Covid-19 in Madrid by Nikolas Bernaola et al was submitted to the medRxiv medical preprint archive in July 2020 (current peer review status unknown). It concluded: “In this multicenter study of patients admitted with COVID-19, hydroxychloroquine and prednisone administration was found to be associated with improved outcomes. Other treatments were associated with no effect or worse outcomes.” However, it sounds a warning note, “Randomized, controlled trials of these medications in patients with COVID-19 are needed to avoid heavy administration of treatments with no strong evidence to support them.”
Tarek Sulaiman’s Team, Riyadh, Saudi Arabia
Placed on the medRxiv preprint archive on 13 September 2020 with the names of 22 authors, “The Effect of Early Hydroxychloroquine-based Therapy in COVID-19 Patients in Ambulatory Care Settings: A Nationwide Prospective Cohort Study”. From the Abstract,
“This observational prospective cohort study took place in 238 ambulatory fever clinics in Saudi Arabia, which followed the Ministry of Health (MOH) COVID-19 treatment guideline. This guideline included multiple treatment options for COVID-19 based on the best available evidence at the time, among which was Hydroxychloroquine (HCQ). Patients with confirmed COVD-19 (by reverse transcriptase polymerase chain reaction (PCR) test) who presented to these clinics with mild to moderate symptoms during the period from 5-26 June 2020 were included in this study. Our study looked at those who received HCQ-based therapy along with supportive care (SC) and compared them to patients who received SC alone.
All patients were presenting with active complaints; however, the HCQ groups had higher rates of symptoms compared to the SC group (fever: 84% vs 66.3, headache: 49.8 vs 37.4, cough: 44.5 vs 35.6, respectively). Early HCQ-based therapy was associated with a lower hospital admission within 28-days compared to SC alone (9.4% compared to 16.6%, RRR 43%, p-value <0.001). The composite outcome of ICU admission and/or mortality at 28-days was also lower in the HCQ group compared to the SC (1.2% compared to 2.6%, RRR 54%, p-value 0.001). Adjusting for age, gender, and major comorbid conditions, a multivariate logistic regression model showed a decrease in the odds of hospitalisation in patients who received HCQ compared to SC alone (adjusted OR 0.57 [95% CI 0.47-0.69], p-value <0.001). The composite outcome of ICU admission and/or mortality was also lower for the HCQ group compared to the SC group controlling for potential confounders (adjusted OR 0.55 [95% CI 0.34-0.91], p-value 0.019).
CONCLUSION Early intervention with HCQ-based therapy in patients with mild to moderate symptoms at presentation is associated with lower adverse clinical outcomes among COVID-19 patients, including hospital admissions, ICU admission, and/or death.”
Derwand, Scholz and Zelenko (USA Data)
The December 2020 edition of the International Journal of Antimicrobial Agents contains the peer reviewed paper “COVID-19 outpatients: early risk-stratified treatment with zinc plus low-dose hydroxychloroquine and azithromycin: a retrospective case series study”. The Abstract reads,
“The aim of this study was to describe the outcomes of patients with coronavirus disease 2019 (COVID-19) in the outpatient setting after early treatment with zinc, low-dose hydroxychloroquine and azithromycin (triple therapy) dependent on risk stratification. This was a retrospective case series study in the general practice setting. A total of 141 COVID-19 patients with laboratory-confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in the year 2020 were included. The main outcome measures were risk-stratified treatment decision and rates of hospitalisation and all-cause death. A median of 4 days [interquartile range (IQR) 3–6 days; available for n = 66/141 patients] after the onset of symptoms, 141 patients (median age 58 years, IQR 40–67 years; 73.0% male) received a prescription for triple therapy for 5 days. Independent public reference data from 377 confirmed COVID-19 patients in the same community were used as untreated controls. Of 141 treated patients, 4 (2.8%) were hospitalised, which was significantly fewer (P < 0.001) compared with 58 (15.4%) of 377 untreated patients [odds ratio (OR) = 0.16, 95% confidence interval (CI) 0.06–0.5]. One patient (0.7%) in the treatment group died versus 13 patients (3.4%) in the untreated group (OR = 0.2, 95% CI 0.03–1.5; P = 0.12). No cardiac side effects were observed. Risk stratification-based treatment of COVID-19 outpatients as early as possible after symptom onset using triple therapy, including the combination of zinc with low-dose hydroxychloroquine, was associated with significantly fewer hospitalisations.”
Brazil
Published online in October 2020 in Travel Medicine and Infectious Disease the peer reviewed paper “Risk of hospitalization for Covid-19 outpatients treated with various drug regimens in Brazil: Comparative analysis” carried the names of 9 Brazilian authors and one from the USA. From the Abstract,
“Use of hydroxychloroquine (HCQ), prednisone or both significantly reduced hospitalization risk by 50–60%. Ivermectin, azithromycin and oseltamivir did not substantially reduce risk further….This work adds to the growing literature of studies that have found substantial benefit for use of HCQ combined with other agents in the early outpatient treatment of COVID-19, and adds the possibility of steroid use to enhance treatment efficacy.”
Switzerland’s Natural Experiment
A rather stunning piece of evidence for hydroxychloroquine’s effectiveness comes from Switzerland, as explained in this article: “Covid-19: l’hydroxychloroquine marche, une preuve solide?” My French is not good, but I get “une preuve solide” right enough. Some background is required first.
On 22 May 2020, The Lancet published a paper which has become scandalous. It claimed that hydroxychloroquine or chloroquine when used for treatment of COVID-19 decreased in-hospital survival rates. It met with a maelstrom of criticism, and was retracted by The Lancet just 13 days later on 4 June 2020. This is a most unusual occurrence. Worse still, it would appear not simply to be procedurally poor, wrong or incompetent, but fraudulent. The newspaper FranceSoir actually uses the term “fraudulent study” (l’étude frauduleuse). It would seem its database did not actually exist.
But in the 13 days before the paper was withdrawn it did its intended damage: the WHO and many Nation States decided to ban the prescribing of hydroxychloroquine due to the scare caused by that paper. (The REMAP-CAP trial of hydroxychloroquine was terminated due to it). Switzerland, in which hydroxychloroquine had previously been in widespread use, was one of the countries which introduced a ban – on 27 May. But, after The Lancet paper had been withdrawn, the Swiss rescinded the ban and the widespread prescribing of hydroxychloroquine restarted after 15 days on 11 June. This created a natural experiment of the efficacy of hydroxychloroquine. The result is shown by Figure 1 below.
Figure 1
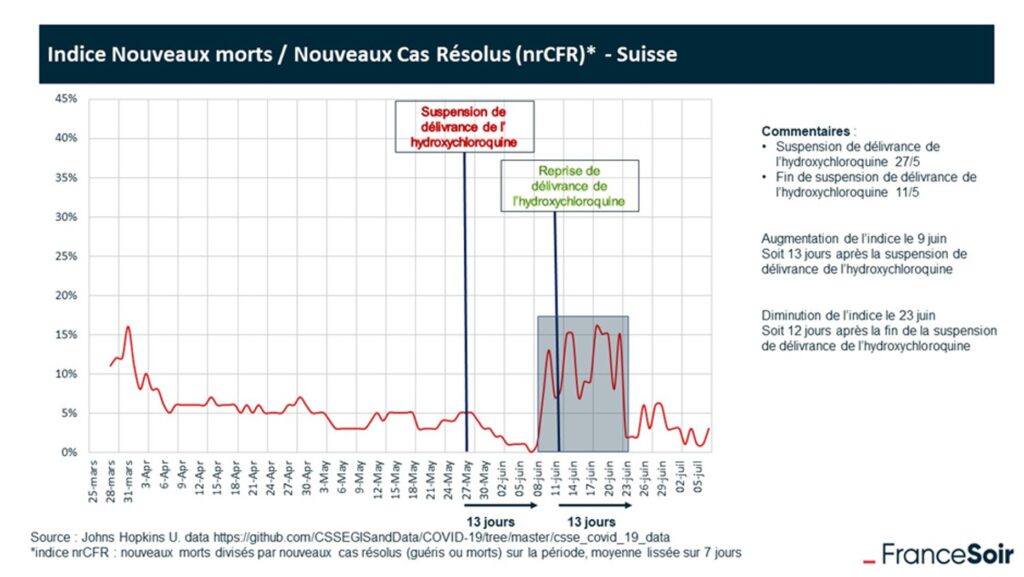
The graph shows, in effect, the mortality rate of Covid-19 patients against date. To be precise, the ordinate is the nrCFR (New Resolved Case Fatality Rate), which is defined as the proportion of deaths among the resolved cases (dead or cured) in the 7 days preceding the date in question.
The two vertical lines indicate the cessation of prescribing hydroxychloroquine (27 May) and its reintroduction 15 days later (11 June). Prior to 27 May, hydroxychloroquine was in widespread use and the death rate was reducing. A time delay between treatment change and any influence on death rate is to be expected. 13 days after the ban on hydroxychloroquine, the death rate leapt upwards to a level not seen since March. 13 days after the reintroduction of hydroxychloroquine, the death rate dropped equally precipitously back down to its previous low level. Une preuve solide, it would seem.
Stunning though that is, there is evidence perhaps even more stunning.
Covid Analysis Group
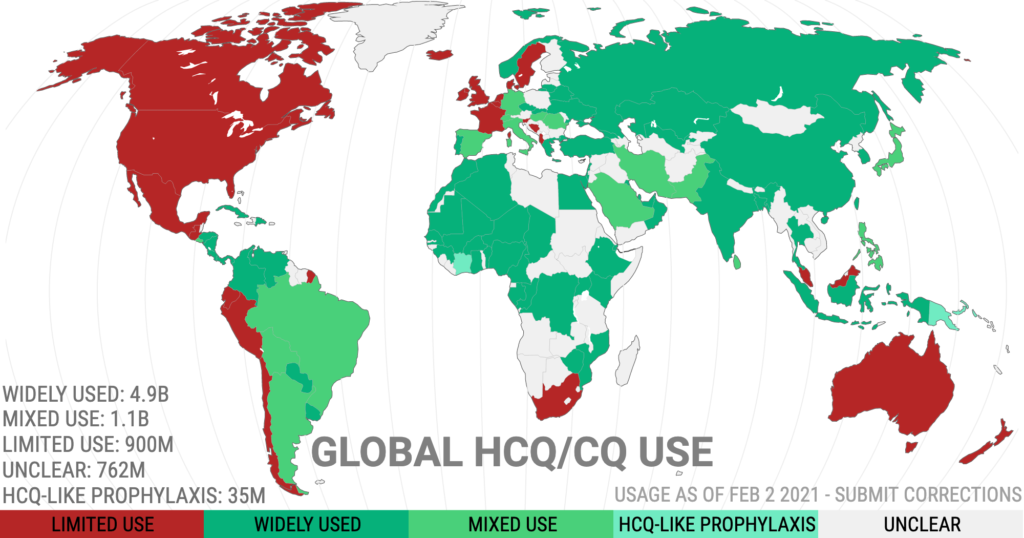
I had it in mind to look at how the overall death rates varied between countries according to their usage of hydroxychloroquine. But it’s been done already. A group of researchers calling themselves Covid Analysis Group has been publishing regular updates of exactly that data, the latest being 14 November 2020: Early treatment with hydroxychloroquine: a country-based analysis. The key result is shown in Figure 2. The analysis is very detailed. Figure 2 includes adjustments described in the web link, which also gives the raw data. I have not attempted to critique or reconstruct the data.
Figure 2 shows in green the death rate per million for countries with widespread early usage of hydroxychloroquine. The red lines relate to countries with limited early usage of hydroxychloroquine. The graph is so striking it needs no extra commentary: virtually all the green countries lie at lower death rates than virtually all the red countries. Note the stipulation regarding the deployment of hydroxychloroquine in the early stages of the disease.
Figure 2:
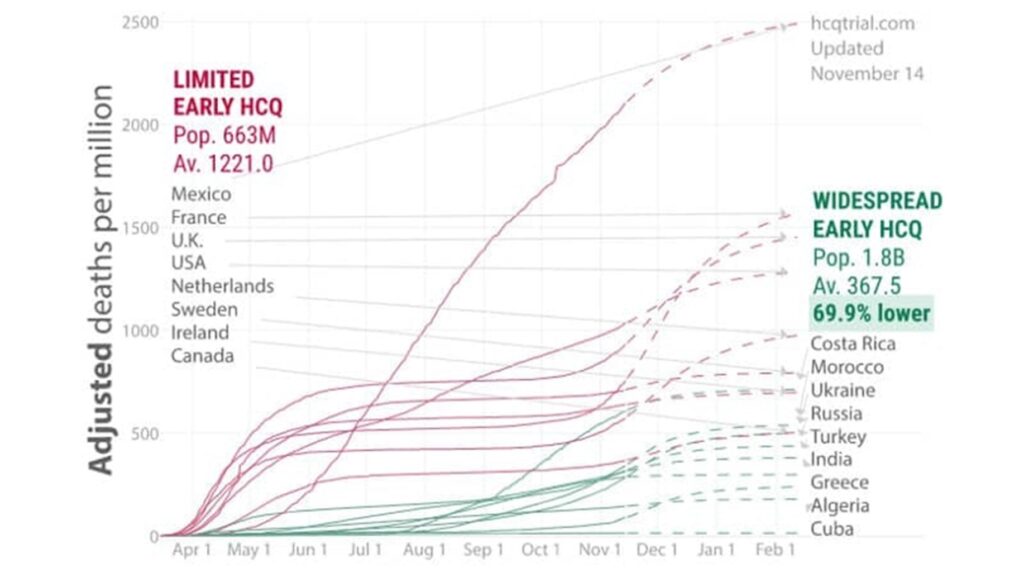
The most obvious concern is whether the countries plotted have been cherry-picked, since only a small sub-set of the world’s nations are included. Covid Analysis Group address the issue thus: “Why is country x not included? Our goal is to identify countries that have taken a strong decision on treatment. Countries without clear decisions are much harder to analyze – to create any meaningful results we need to know the proportion of usage to some reasonable degree….Countries like Italy or Brazil have extremely mixed usage, with differences during major time periods of their outbreak and/or major geographic differences. Analyzing these countries would be much more complex.”
The Totality of Available Studies at 26th February 2021
As of 26th February there are 259 studies, 187 peer reviewed, and 211 which compare with control groups. You can find them all listed on this very useful link. I quote that site’s summary of the totality of evidence,
“HCQ is not effective when used very late with high dosages over a long period (Recovery/Solidarity), effectiveness improves with earlier usage and improved dosing. Early treatment consistently shows positive effects. Negative evaluations typically ignore treatment time, often focusing on a subset of late stage studies.”
Figures 3, 4 and 5 below are lifted straight from that source. Figure 3 shows that all 27 studies which involved treatment during the early stage of the disease were efficacious, the average improvement being 65%. Figure 4 shows all the trials, on both early and late stage patients. Most show a beneficial effect, but a substantial number of late-stage studies produce a worse outcome than controls. Early stage treatment is therefore key, as has been consistently maintained by proponents of hydroxychloroquine in the medical (as opposed to research) community.
Figure 5 shows the distribution around the world of usage of hydroxychloroquine to treat Covid-19. Limited use is found across all anglophone countries. But I suggest there may be an even stronger correlation with the distain for hydroxychloroquine: politics.
Figure 3
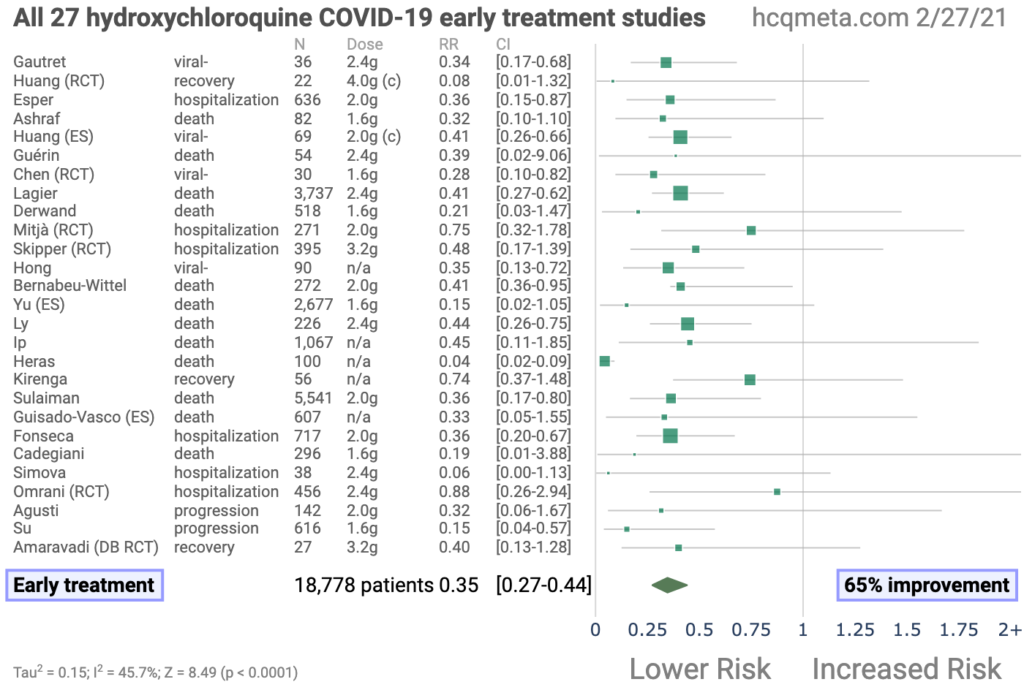
Figure 4
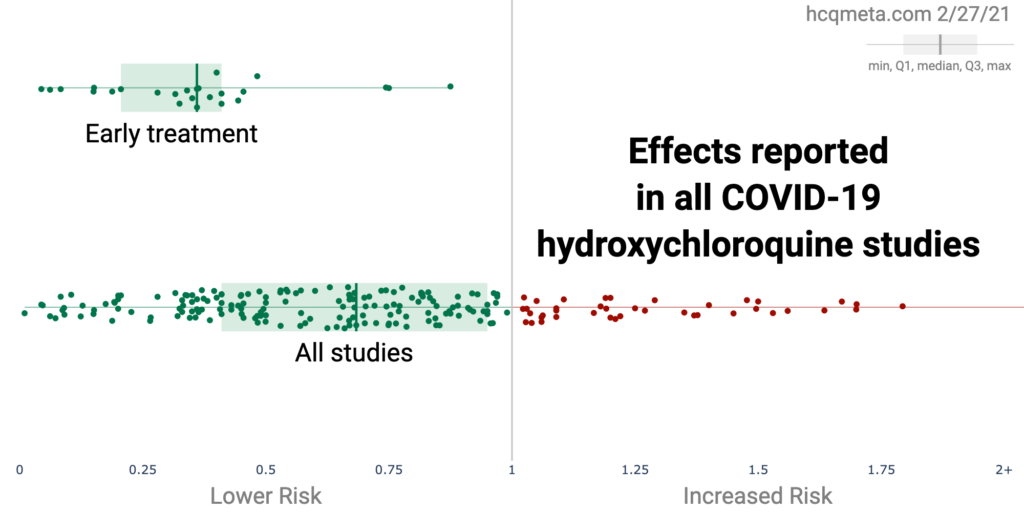
Figure 5
Has the Efficacy of Hydroxychloroquine been Misrepresented?
So, has much of the world failed to benefit from an effective, early-stage treatment for Covid-19, because early trials of hydroxychloroquine were misleading? Based on the evidence now accumulating it would appear so. But one of the surprises, to this author, is that the negative view of the efficacy of hydroxychloroquine is highly country-specific, being universal across anglophone countries, whilst most other countries have continued to deploy it.
One worries that this might be a result of Trump Derangement Syndrome, which is very real and induces a compulsion to discredit anything associated with the name of Trump. That President Trump aligned himself with hydroxychloroquine at one time may have been sufficient motivation for some people to be determined, not merely to discredit it, but preferably to heap ridicule upon it too.
The possibility that such political partisanship might have caused some to be overly eager to discredit hydroxychloroquine is discomforting. The suspicion that a year of lockdowns, with all its ramifications, could have been avoided but was embraced due to political partisanship is extremely worrying.

Very interesting, thank you. I find it very hard to get reliable data on this area: there are so many interests and the money involved is so vast that the usual swamp of competing information is as bad as global warming or cultural Marxism/feminism, but without the long-term paper trail that can be followed on those topics.
And, just as with most other areas where the media will tout “women most affected” the truth is that men are being discriminated against at every twist and turn.
For every 10 female…
…Tests…there are 8 male Tests
…Cases…there are 11 male Cases
…Hospitalisations…there are 12 male Hospitalisations
…ICU admissions…there are 18 male ICU admissions
…Deaths…there are 13 male Deaths
…Confirmed cases that have died…there are 15 male Confirmed cases that have died
…Vaccinations…there are 9 male Vaccinations
Source: The sex, gender and Covid-19 project.
https://globalhealth5050.org/the-sex-gender-and-covid-19-project/
I was kind of going along with AJ, accusing people of conflict of interest is a bit dubious, until I got to the graphs and slides
damn….
I had been looking at relative death rates in various countries for some time, trying to work out what the heck was going on for them to be so different, now it look pretty clear whats happening.
Check out total deaths in Cameroon from corona virus, hardly any, their government tell them to boil tree bark which produces quinine
I think your concerns are well placed and it’s good to see some light being directed at the shadows that surround the official narrative. There is something very disturbing about governments that prevaricate over treating covid with an established and seemingly efficacious drug while giving almost immediate emergency authorisation to experimental vaccines that have not undergone full testing.
I note that the ‘roadmap’ revealed last week made a couple of references to a second vaccine later this year. I suspect this will be the one that Gates has been telling us about for several months.
https://www.hindustantimes.com/world-news/normalcy-only-when-second-generation-of-covid-19-vaccine-is-out-bill-gates/story-j96EWApnUeW827lfiEORZJ.html
Excellent article. Regarding the motives that you allude to as far as I’m aware all the covid vaccines have been granted emergency authorization by both the UK & US pharma regulators. These emergency authorisations were only legally possible if NO existing treatments were available. If HCQ was proven to be an effective treatment, then the vaccines could not be granted emergency authorisation.
I am really finding this uncomfortable reading. I have always found your articles well argued, persuasvive and grounded. You established, with me at least, a reputation for solid grounded analysis. Unfortunate;y you have thrown it away with this. Please reread what you have written think about whether it enhance sor damage syour reputation and whether you really wnat this on your website.
I have onlyy read so far but:
1. The idea that there is a conflict of interest between one instituition amongst many producing a vacine and an evaluation of possible therapies reads as paranoid nonsense. This sort fo ‘conflict’ is inevitable in any scientific endeavour where there is a limited pool of experts all of whom are to an extent in competiotion. The idea that teh Bill and Melinda Gates foundation are somehow malignly involve dis just crackpot.
2. I stopped reading at the point you were discussing the exponential decay of dosage with an entirely arbitrary factor of 0.8 per day. A value massively different from the easily found biological half life for the drug. It is clear that a simplistic analysis along thes elines is in any case flawed becaus ethe halflife is long and therefore the peak accumulated level from all the regimes described seem high. Thsi just reionfirces that a naive analysis does not make sense. Basing such an analysis on a number plucked from the air makes it nonsense on top of nonsense and in anycase the dosage seems broadly aligned with dosages for other conditions and there is a recognised danger with giving doses much higher.
This absolutely is not my expertise but the trials gave doses which were aligned with the high end of exisiting practice. This seems as reasonable a choice as good be made to try to establish a benefit.
I might be soft but I was genuinely embarassed on your behalf that you published this and stopped reading at that point. Are you really positing a worldwide consipracy of medical experts and researchers to conceal an effective treatment, with no personal benefit to them and greate risk resulting in the depths of hundreds of thousands? Why in this case have the same insituitions and individuals meanwhile help conduct other trials with the same supposed conflicts of interest that results in the identification of effective non-vacine therapies?
I have a lot of sympathy with your criticisms. You might find the article beyond where you stopped reading more convincing, though. I was surprised how much evidence there is in favour of HCQ in early stage patients – and, on reflection, this is the true burden of the article.
The 0.8 decay is indeed arbitrary – and I hope I said that clearly. I was in two minds whether to include it. But I understand there is a degree of cumulative effect so I needed some sort of discount rate. Actually all it really amounts to is that the two trials used doses on days 2 to 10 which are double the recognised maintenance doses (contrary to what you say), and this is reflected in the graph if you ignore the absolute scale. But admittedly it is not clear if the trial doses are indeed in the hazardous range, as this is an artefact of the 0.8 assumed.
[Incidentally, I believe the half life in blood is 537 hours, and far longer in plasma. The shorter figure is equivalent to 0.969 in 24 hours. But I believe the elimination time is not the controlling thing here, but the distribution time. I may be wrong and am happy to be educated, but that’s why I didn’t use elimination half-life. It would imply an alarmingly high effective dose (>13 g) to result from a long-term maintenance dose of 400 mg, which can’t be right].
The issue of conflict of interest seems real to me. What is normal practice in this area? I can’t believe a shortage of suitably qualified people was the reason. But adverse results from a conflict of interest do not imply a cold blooded conspiracy of wicked villains. It can operate in more subtle ways, through a willingness to believe what one wants to believe. Unfortunately, very bad things can result from the best of intentions. Admittedly I’ve mudded the water by straying into polemic like “deliberately scuppering trials” and “crime against humanity”.
Also, the Mehra et al Lancet article debacle is disturbing, especially as countries around the world reacted within days to that one article to suspend HCQ usage/trials.
But the real issue is the accumulating weight of evidence of the effectiveness of HCQ when used as its proponents always intended, as an early intervention. Is this now too politically inconvenient to ever surface as common knowledge? That is, I think, the point I’m really making….that as far back as March an effective treatment for the early stage illness existed and could have been rolled out to reduce, perhaps very dramatically, the numbers of people going into hospital, removing the threat of overwhelming the NHS, reducing the death rate, and perhaps avoiding lockdowns…at least beyond April.
As for reputational damage – yes, I did consider that. Until a few days ago I would not have posted on HCQ or any other Covid treatment. But I was alarmed at what I found when I looked. Of course I may be wrong.
What a strangely limp-wristed response to a barely literate attack on your integrity! I suspect you are inclined to be overly respectful of correspondents who can throw a bit of scientific analysis back at you. Your critic’s condemnation of your own analysis is wantonly purblind to accepted data. HDCHQ, when used early, has proven success in combatting Sars-2-Covid. Moreover, your critic’s counter assertion that Big-Pharma and its scientists are morally squeaky clean and impervious to the charge of politicking is so ludicrous as to beggar belief. One has but to recall the Channel Four television exposé of their malfeasance over another non-existent ‘pandemic’, i.e., that of so-called ‘swine flu’ back in 2010 to realise just how capable such people are of acting in their own criminal self-interest. Sars-2-Covid has an average lethality age of 82.2 years old. It is 99.96% survivable, even among those who do contract it. It was classed as a pandemic only because the morally defunct, Marxist WHO lowered the classification bar to make it one. As for the Gates Foundation, its ban from operating in India for violating basic standards in vaccine trials is common knowledge. Gates is a class-A megalomaniac posing as a philanthropist. He is unelected so he buys power with his billions and exercises it in ways that are accountable to no-one but himself and his weird wife. Witness their maniacal support of the Climate alarmism agenda. They should be held to account, but of course won’t be because money talks louder than ever in our ethically bankrupt age. As for Covid, I shared the public’s initial naivety over its potential severity back in March 2020. I now see that this was sadly mistaken. Serious for some, fatal for a tiny percentage (proportionate to the world population), Covid is an epidemiological non-event for the overwhelmingly vast majority of mankind. In this context it is a tragic scandal of international proportions that Covid has been used as a screen to usher in The Great Reset and the rule of the Technocrats. Would that the latter were only a ridiculous conspiracy theory. Sadly it cannot be dismissed thus precisely because they themselves are openly telling us (on their various websites, WHO, WEF and UN 2020 agenda, etc) that they’re doing it! Ask yourself: was it Covid that wrecked our culture – or our corrupt politicians? Is it too late to stop the total annihilation of our whole way of life. Probably. It’s already past midnight and I see no sign of intelligence about this scandal dawning in the public mind.